Targeting cystatin F activation enhances NK cell cytotoxicity in glioblastoma models
- Post by: Jure Pohleven
- 4.February, 2026
- Comments off
A recent study by the Cancer Proteolytic Pathways Research Group addresses a key reason why cancer remains difficult to treat: many tumours establish a profoundly immunosuppressive microenvironment that compromises anti-tumour immunity. Among the most promising effector populations are natural killer (NK) cells, which can efficiently recognize and eliminate cancer stem cells—a tumour subpopulation often resistant to standard therapies and implicated in recurrence. However, in patients, NK-cell cytotoxic function is frequently impaired, indicating active suppression by the tumour and its associated microenvironment.
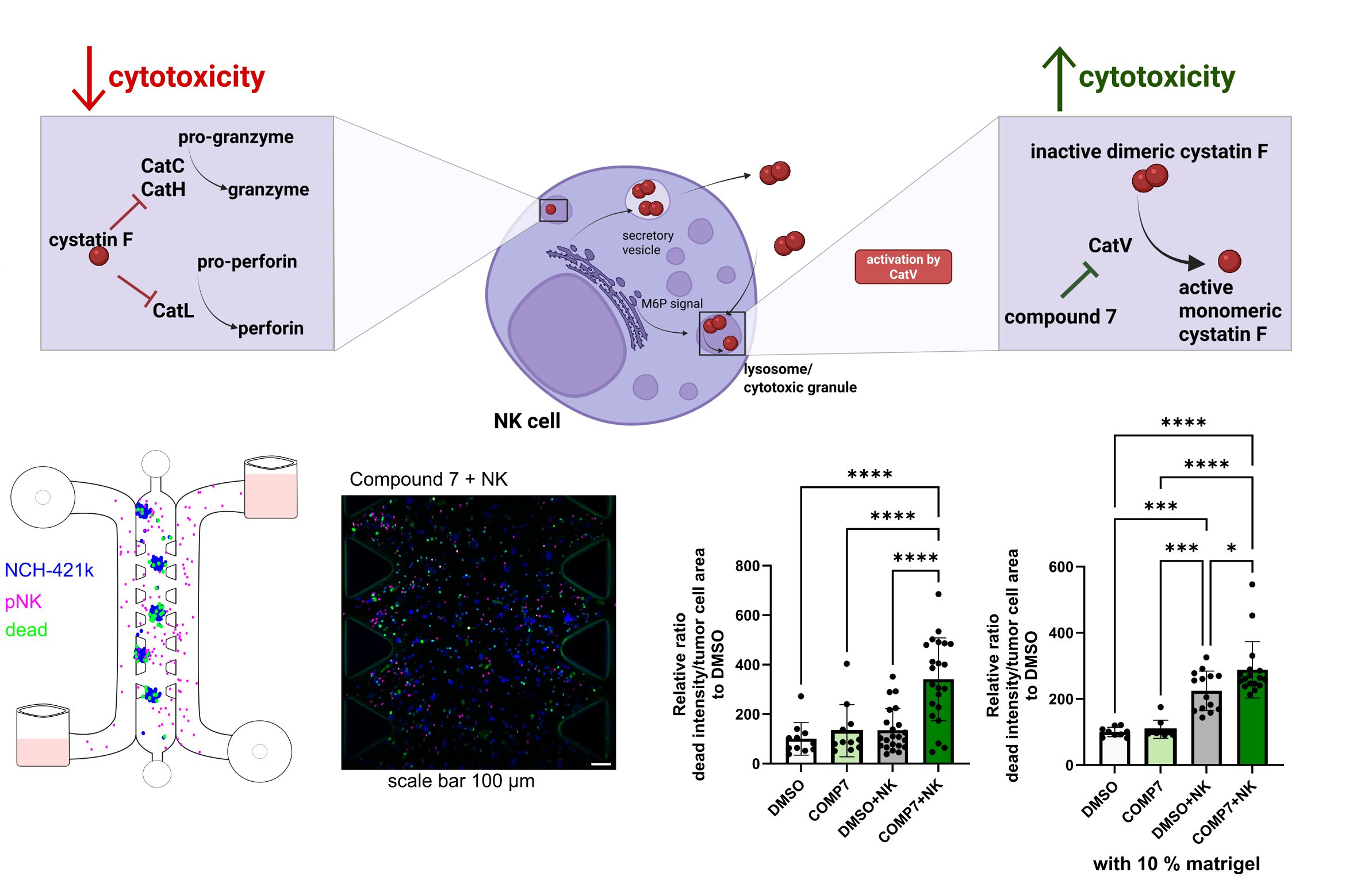
Our previous work identified the protease inhibitor cystatin F (CST7) as an important mediator of this impairment. CST7 expression correlates with markers of immune exhaustion, and we demonstrated that cystatin F–positive microglia can directly suppress NK-cell activity, thereby reducing their capacity to kill malignant targets. A major conceptual advance was the recognition that cystatin F is regulated through an activation switch: the inactive dimer is converted into the active monomer by cathepsin V. This mechanistic insight enabled a targeted pharmacological intervention—rather than broadly “stimulating immunity,” we aimed to disrupt a specific pathway responsible for NK-cell suppression.
Accordingly, we applied a selective cathepsin V inhibitor, developed in collaboration with the Faculty of Pharmacy, and showed that this approach restores NK-cell cytotoxicity. Importantly, the effect was validated not only in standard functional assays but also in more physiologically relevant systems, including 3D tumour spheroids and microfluidic perfusion models, where controlled flow better mimics tissue-like conditions and thereby enhances the translational relevance of the findings. This progression from conventional 2D assays to 3D and microfluidic platforms is critical for assessing whether observed effects persist under conditions that more closely recapitulate the tumour milieu in vivo.
The study, published in Frontiers in Immunology (IF = 5.9), was led by researchers from the Department of Biotechnology (Dr Emanuela Senjor, Dr Ana Mitrović, Dr Janko Kos, and Dr Milica Perišić Nanut) in collaboration with colleagues from University Medical Centre Ljubljana, the Faculty of Pharmacy, and the National Institute of Biology (NIB). Overall, the work exemplifies effective integration of fundamental immunology, clinical samples, and advanced disease models (3D culture and microfluidics) to enable more realistic preclinical analyses and to support the development of improved strategies to overcome tumour-driven immunosuppression in cancer.
Link to the publication: https://doi.org/10.3389/fimmu.2025.1708281

