Proteolytic system in cancer
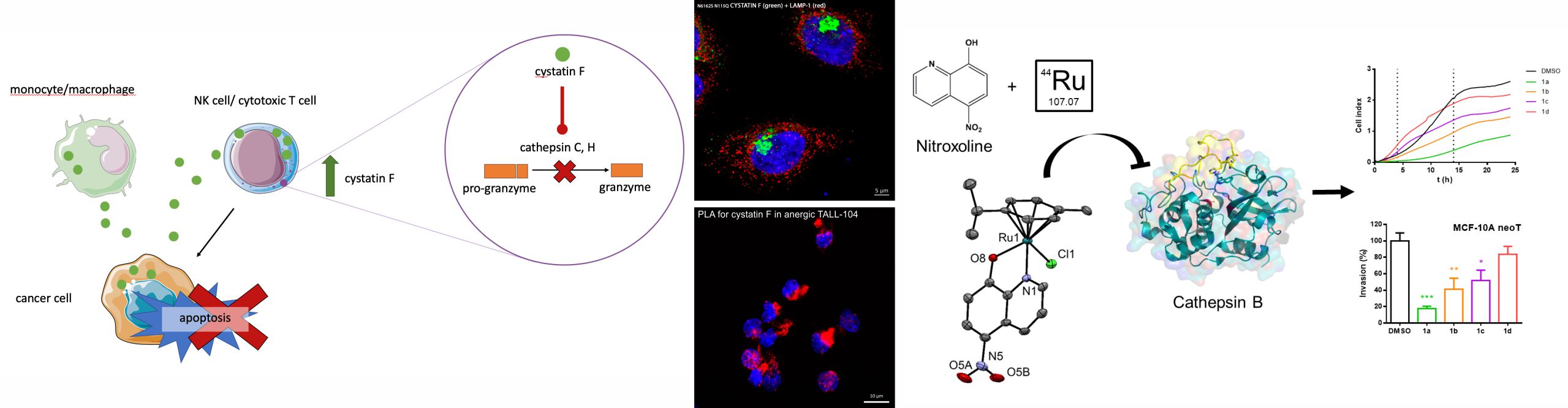
Irregular function of the proteolytic system represents a hallmark of various diseases, including cancer, immune and neurological disorders. For many years our research group investigates the involvement of various peptidases and their inhibitors in the molecular mechanisms leading to the progression of these diseases with aim to identify the targets for development of new diagnostic and therapeutic strategies. As important factors we characterized cysteine cathepsins, in particular cathepsins B and X. To impair their harmful pathological activities a set of specific inhibitors has been developed and proved to reduce tumor growth and metastasis in vitro and in vivo. Cysteine peptidases are important also in regulation of antitumor immune response, enabling tumor antigen presentation to T and B lymphocytes, promoting immune cell adhesion and migration, affecting the activity of chemokines, and through the activation of granzymes providing cytotoxicity of T lymphocytes and natural killer (NK) cells. A significant part of our investigations is focused on interactions between tumor and immune cells in the tumor microenvironment. In this respect, the protein inhibitor of cysteine peptidases cystatin F is an important mediator. It impairs the activation of granzymes, peptidases, found in the secretory granules of cytotoxic cells by inhibiting cathepsins C and H, which convert progranzymes into active forms. Active granzymes enter the target cells and trigger cell killing by apoptosis. Tumor cells and immune cells in the tumor microenvironment can secret the inactive dimeric form of cystatin F, which is then internalized into cytotoxic cells and, after activation, inhibits granzyme processing. We proved that the cytotoxicity of NK cells and cytotoxic T lymphocytes is directly related to the level of active cystatin F. To impair the function of cystatin F in cytotoxic cells we intend to improve their application in cancer immunotherapy.
Selected publications
- PERIŠIĆ, Milica, PAWELEC, Graham, KOS, Janko. Human CD4+ T-cell clone expansion leads to the expression of the cysteine peptidase inhibitor cystatin F. International journal of molecular sciences, 2021, 16, 8408-1-8408-15.
- SENJOR, Emanuela, PERIŠIĆ, Milica, BREZNIK, Barbara, MITROVIĆ, Ana, MLAKAR, Jernej, ROTTER, Ana, PORČNIK, Andrej, LAH TURNŠEK, Tamara, KOS, Janko. Cystatin F acts as a mediator of immune suppression in glioblastoma. Cellular oncology, 2021, 13.
- PRUNK, Mateja, PERIŠIĆ NANUT, Milica, JAKOŠ, Tanja, SABOTIČ, Jerica, ŠVAJGER, Urban, KOS, Janko. Extracellular Cystatin F Is Internalised by Cytotoxic T Lymphocytes and Decreases Their Cytotoxicity. Cancers, 2020, 12(12), 3660.
- MITROVIĆ, Ana, ZAVRŠNIK, Janja, MIKHAYLOV, Georgy, KNEZ, Damijan, PEČAR FONOVIĆ, Urša, MATJAN-ŠTEFIN, Petra, BUTINAR, Miha, GOBEC, Stanislav, TURK, Boris, KOS, Janko. Evaluation of novel cathepsin-X inhibitors in vitro and in vivo and their ability to improve cathepsin-B-directed antitumor therapy. Cellular and molecular life sciences. 2022, 79, 1, 34-1-34-14.
- MITROVIĆ, Ana, KLJUN, Jakob, SOSIČ, Izidor, URŠIČ, Matija, MEDEN, Anton, GOBEC, Stanislav, KOS, Janko, TUREL, Iztok. Organoruthenated nitroxoline derivatives impair tumor cell invasion through inhibition of cathepsin B activity. Inorganic chemistry, 2019, 58, 18, 12334-12347.


